Personal Genome Diagnostics Inc. (PGDx) announced that PGDx elio plasma resolve has received Breakthrough Device designation from The Center for Devices and Radiological Health (CDRH) of the U.S. Food and Drug Administration (FDA). PGDx elio plasma resolve is a qualitative in vitro diagnostic test that uses targeted high throughput, parallel-sequencing technology to detect single nucleotide variants (SNVs), small insertion/deletions (indels), …
-
Houston Methodist Opens New State-Of-The-Art Patient Tower In Texas Medical Center...
-
MiMedx Receives Regulatory Approval to Commercialize Product Portfolio in Australia...
-
NuVasive and Siemens Healthineers partner to transform spine surgery...
-
Boston Scientific Announces Agreement to Acquire VENITI, Inc....
-
CHA-Hollywood Presbyterian Medical Center at the Forefront of Robotic Surgical Technology...
-
Abbott Begins U.S. Pivotal Trial for the Tendyne Mitral Valve Replacement System to Treat Patients with Heart Valve Disease...
-
Welltower And ProMedica Complete Acquisition Of Quality Care Properties And HCR ManorCare For $4.4 Billion...
-
Perflow Medical Announces the First Clinical Use of the Cascade Dynamic Non-Occlusive Remodeling Net...
Karmanos Expands Cancer Network with Newest Location at McLaren Oakland
The Barbara Ann Karmanos Cancer Institute and McLaren Oakland announced today it will open its 15th treatment location in Michigan at McLaren Oakland in Pontiac next month. “McLaren Oakland will soon be home to a new 21-bed inpatient state-of-the art oncology unit,” said Margaret Dimond, Ph.D., president and chief executive officer, McLaren Oakland. “Karmanos Cancer Institute is one of the …
Taiwan Unveils Latest Medical Inventions to Advance Science During FIME in Orlando
Health Execs, physicians and diplomats were awed by the new medical devices presented by three Taiwanese companies that will enhance surgeon skills, accurately detect thyroid cancer and relieve patient bed sores. “Taiwan Medical Marvels” was the theme of this international press conference and products launch held at the Orange County Convention Center in Orlando during the Florida International Medical Expo (FIME), one of the …
Northwestern Medicine Central DuPage Hospital Earns Prestigious Lantern Award
The Emergency Nurses Association will bestow the Northwestern Medicine Central DuPage Hospital Emergency Department with its prestigious Lantern Award. The department is one of only 19 Emergency Departments in the U.S. — and the only one in Illinois — to receive the award for this three-year cycle (2018 – 2021). The Emergency Nurses Association (ENA) Lantern Award recognizes emergency departments …
Cedars-Sinai Accelerator Start-Ups Aim to Speed Solutions to Market
Health-Tech Companies in Latest Cedars-Sinai Class Seek to Address Pressing Healthcare Needs, Including Health System Efficiency and Patient Engagement, Through AI and Other Technologies Nine health-tech companies have joined the newest class of the Cedars-Sinai Accelerator as they look to develop and refine solutions to some of healthcare’s most pressing challenges, particularly those affecting the experience of patients. The companies, …
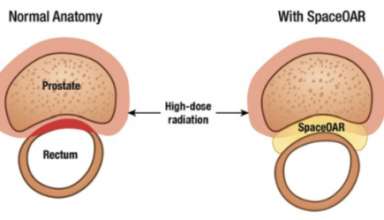
New Radiotherapy Prostate Cancer Treatment, SpaceOAR Hydrogel, Now Available in Japan
Augmenix K.K. announces that SpaceOAR hydrogel, a soft, implanted absorbable gel spacer is now available to all prostate cancer radiotherapy patients in Japan through the Ministry of Health, Labour & Welfare (MHLW) national reimbursement. SpaceOAR hydrogel is clinically proven to assist in the decrease of unwanted side effects from radiotherapy treatment in patients with prostate cancer. Stephen J. McGill, Vice …
Life Spine Announces Key Initiative around Micro-Invasive Procedural Solutions and Robotic Assisted Surgeries in an Ambulatory Surgery Center
Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders announced the first surgeries with robotic assisted minimally disruptive placement of the CENTERLINE® Cortical Screw System and the PROLIFT® Expandable Spacer System in an ambulatory surgical center. “Life Spine is dedicated to address and support minimally disruptive technologies such as …
Intuitive Surgical Receives FDA Clearance for First 60mm Stapler
Intuitive Surgical announced that the U.S. Food and Drug Administration granted clearance for the company’s fully wristed, 60mm stapler. SureForm 60™, a single-patient use 60mm stapler, offers 120 degrees of fully wristed articulation – an industry first. Surgeons control SureForm 60 through the da Vinci surgeon console, creating an immediate connection between clinical decision-making and instrument action. Combining da Vinci’s tremor filtration …
Boston Scientific to Acquire Cryterion Medical
Boston Scientific Corporation announced a definitive agreement to acquire Cryterion Medical, Inc., a privately-held company developing a single-shot cryoablation platform for the treatment of atrial fibrillation (AF). The addition of this cryoballoon platform positions the company as the first to have both cryothermal and radiofrequency (RF) single-shot, balloon-based ablation therapies in its portfolio. Boston Scientific has been an investor in …
Advanced Instruments Introduces OsmoTECH Single-Sample Micro-Osmometer

Advanced Instruments announced the release of the OsmoTECH Single-Sample Micro-Osmometer. OsmoTECH’s data management capabilities, security features, and embedded web server far surpass the capabilities of other osmometers on the market today. OsmoTECH provides the 21 CFR Part 11 compliance features that GMP environments demand. Plus, the system uses freezing point depression—the gold-standard testing method in osmometry—to provide the accuracy and …
Recent Posts
- Houston Methodist Opens New State-Of-The-Art Patient Tower In Texas Medical Center
- MiMedx Receives Regulatory Approval to Commercialize Product Portfolio in Australia
- NuVasive and Siemens Healthineers partner to transform spine surgery
- Boston Scientific Announces Agreement to Acquire VENITI, Inc.
- CHA-Hollywood Presbyterian Medical Center at the Forefront of Robotic Surgical Technology









Recent Comments